Validated Approach for Regulatory Success

Validated Methodology in the UK and the European Union
Our methodology has been successfully validated by food and health authorities in both the UK and the EU. The approach focuses on the systematic collection and integration of historical evidence for foods, food ingredients, herbs and botanicals. This includes records of preparation, consumption context, and safety data spanning multiple generations and countries. By drawing on archived materials, ethnobotanical sources, well-documented food, supplements and herbal use, and original surveys, we build comprehensive evidence packages tailored to meet regulatory expectations.
This evidence has been used to demonstrate significant history of consumption, helping to meet criteria under UK and EU regulations. The outcome has enabled product approvals and licensing for novel and traditional products — showing how a strong historical foundation combined with modern methodologies can open the door to market access.
Evidence Supporting Regulatory Applications
We support clients with scientific dossiers that help substantiate product safety and support health-related or medicinal claims. Our work includes structured assessments of historical consumption and use, identifying potential toxicity concerns, and mapping out risk-benefit profiles to align with EU and UK regulatory standards.
Our team also assists with substantiating traditional use claims, providing the background necessary for botanical food and supplement approvals. These insights are invaluable in product development pipelines, enabling companies to revive forgotten products, innovate using heritage foods, and comply with traditional-use frameworks.
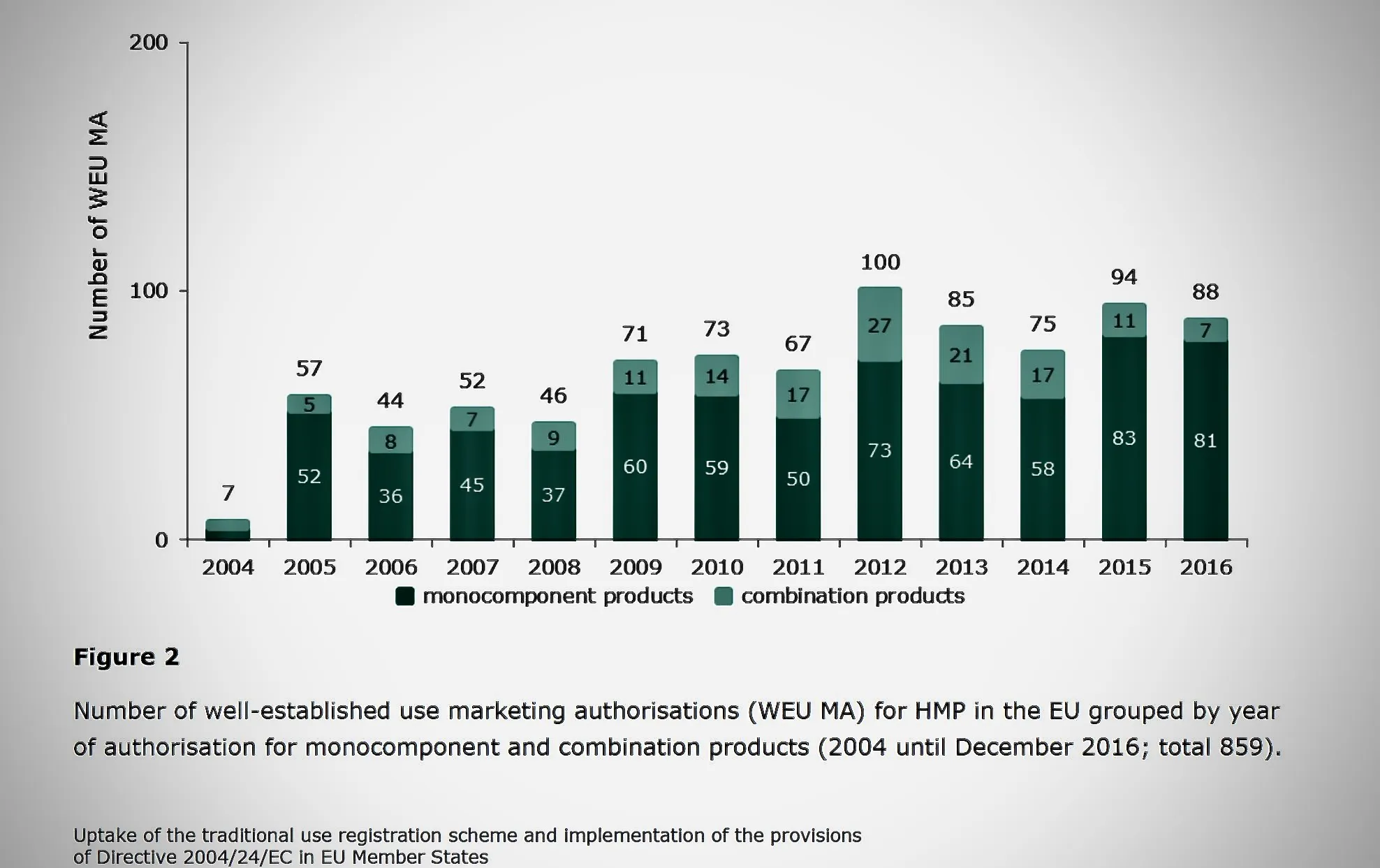
Understanding Market Behaviour and Licensing Options
In addition to safety and claims support, we provide insights into evolving consumer behaviours through market and cultural analysis. This allows clients to align with target demographics and explore strategic positioning for both legacy and innovative products. We also offer flexible licensing options — including access to in-house survey modules and datasets — to help companies scale or replicate research in other regions.
Contact
Let’s explore how our validated research methods can support your next regulatory submission. We work with flexible timelines and rapid response teams.
